ASPHYXIA DOES NOT INJURE THE
BRAIN
[A] Myers
RE. Perinatal brain damage. American Journal of Obstetrics and
Gynecology 1972 112:246-276.30 [A]
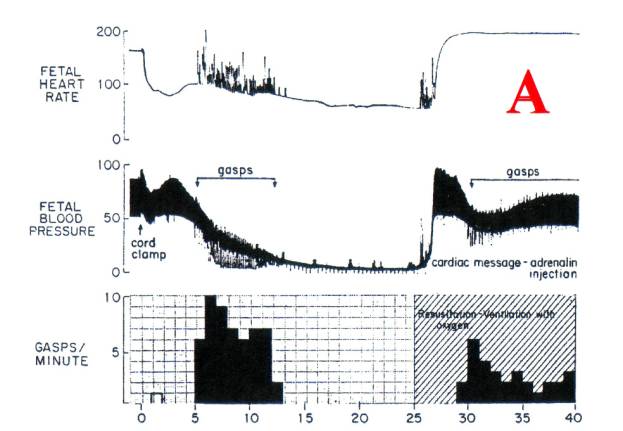
Tracing
[A] is of primate newborn delivered by c-section
and immediately asphyxiated. The cord
was clamped immediately and the head covered with a rubber bag. The eventual result was death; autopsy
showed widespread infarction of brainstem nuclei similar to that seen following
recovery from cardiac arrest.
Brain
infarction results from loss of blood perfusion as in arterial occlusion
(segmental infarct) or cardiac arrest (widespread infarcts of metabolically
active areas.) The fetal brain grows
and develops on a copious supply of partially oxygenated blood; if well
perfused, brain tissue tolerates hypoxia.
However,
myocardial contraction (blood pressure) depends on aerobic
respiration. Under complete
asphyxia [A] heart function gradually fails, the
tissue perfusion force (blood pressure) gradually disappears and brain
infarction begins.
1.
Hypoxic heart
failure causes loss of perfusion.
2.
Loss of perfusion
causes neuron necrosis.
Primate
model [A] does not completely replicate the
human newborn that develops ischemic neuron necrosis. (HIE or NE) Intrapartum hypoxia /asphyxia is a frequent
feature of HIE/NE, [10][15] but no human neonate is subjected to the
degree of anoxia and hypoxic heart failure seen in [A]. Most HIE victims are not (and never were)
significantly hypoxic; however, they all exhibit signs of heart failure,
inadequate tissue perfusion and multi-organ failure, and typical ischemic
neuron necrosis.
·
The prime causal
agent in HIE/NE responsible for loss of perfusion is not hypoxia.
The most common cause of birth asphyxia is cord
compression (e.g. a tight nuchal cord) that impedes oxygen supply to the fetus;
it also acts as a venous tourniquet, impedes venous blood return, engorges the
placenta and thus exsanguinates the fetus.
The severely cord-compressed child is born limp, areflexic, and ashen
white with some blue/black mottling. Routine
treatment is immediate cord clamping (ICC) and ventilation.
·
The child is
hypoxic and EXTREMELY hypovolemic, much of its blood volume is in the engorged
placenta.
Ventilation establishes pulmonary blood flow, and the blood
volume required to perfuse the lungs is drawn from the systemic circulation;
this accentuates the systemic hypovolemia.
The child may stabilize in this state, but in many cases deterioration
is indicated by an important symptom of extreme hypovolemia retraction
respiration. (RR)
RETRACTION RESPIRATION
Gasping
(RR) appears on [A] when blood pressure falls
below 50 mms Hg. RR is a
reflexive reaction to hypo-volemia, hypotension and low central venous pressure. It generates pulses of negative
intra-thoracic pressure (NIP) that pull blood into the right atrium and
ventricle. It is an extreme response to
forestall cardiac collapse and to fill empty cardiac atria and ventricles. The gasps produce spikes of increase in the
heart rate, and similar spiking decreases in the diastolic blood pressure to
the zero line.
NIP
counteracts aortic arterial pressure during a gasp and reverses the pressure
gradient between the thoracic aorta and peripheral arteries. The collapse of diastolic blood pressure
seen during gasps [A] indicates reversed blood
flow from peripheral arteries (e.g carotid) into the thorax. During these gasps and reverse blood flow
there is no perfusion of brain tissue, and diminished perfusion continues until
the meager cardiac output regenerates arterial blood pressure.
·
A
retracting child is in urgent need of a blood transfusion.
RR
is usually regarded as a symptom of respiratory distress, however, the
appearances of RR in [A] correlate only with
hypotensive heart failure, in the first instance with no oxygen in the lungs,
in the second with the lungs filled with oxygen. This strongly indicates that hypoxia is not the cause of RR and
oxygenation will not cure RR. When RR
is recognized as a reflexic attempt to forestall hypovolemic heart failure by
drawing systemic blood volume into the heart, the origin of neonatal cerebral
ischemia becomes apparent.
·
Therefore the
ischemic lesions of HIE are not of hypoxic origin, but are of hypovolemic,
hypotensive origin.
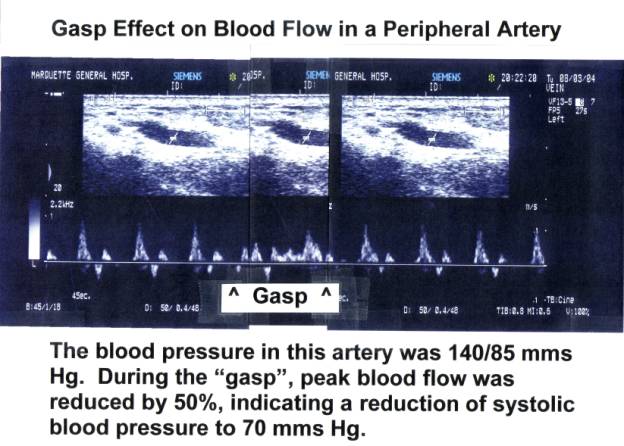
Doppler
studies on carotid blood flow in retracting neonates should readily show
defective brain perfusion when compared to carotid blood flow in neonates that
have received a physiological placental transfusion.
Note:
Cowan [10] there is no
evidence that brain damage occurs before birth. At birth, a pulsating cord indicates that the heart and brain are
being adequately perfused with oxygenated blood. After birth, retraction respiration indicates that the heart is
not adequately perfusing itself or the brain.
The cause of inadequate perfusion and consequent brain damage is blood
loss hypovolemia caused by ICC; asphyxia is not a factor.